Overview
Scientific revolutions are more likely when unexpected experimental outcomes are met with curiosity rather than annoyance. Enabled Therapeutics was founded on luck, which came in the form of a discovery, made possible by curiosity over some puzzling data. Importantly, the discovery implied a singular approach to CNS drug design, one that yokes therapeutic dose to the extent of pathology in situ. Herein lay the revolution. Barely sustained by infrequent fortuity, scientific progress is also hobbled by pride and the inability to venture past accepted truth at different points in history.
Aware of these weaknesses, we recognized the need to share those rare experimental surprises, especially when they contradict our inherited understanding of reality. The surprising discovery, made by Enabled Therapeutics staff in 2016, was illuminated when an old publication describing some long-abandoned seizure medicine was noticed to offer an explanation for some frustratingly inexplicable data, at that moment crumpled into a ball and arcing toward the oblivion of landfill. The solution to our confusion, hinted the old paper inadvertently, was a previously unrecognized phenomenon, the non-enzymatic, stress-dependent, over-oxidation of sulfur (-2 to +4). In this instance, the electron donors would be mercaptan analogs of inhibitory neurotransmitters. The acceptors were reactive oxygen species (ROS). The extracellular space of the brain, one of the few human physiological environments virtually unbuffered against changes in redox potential, was a natural location for the electron transfer to occur. The redox reaction could be accessed, maybe even practically applied, through minor structural modification of a few well-documented, bioactive compounds. We had been accidentally gifted with an “on” switch that allowed dynamic control of drug activity at sites of neuropathology by the pathology itself. Drug empowerment through damage and disease-dependent oxidative functional group exchange, a consequential hypothesis for the future of neurology, began to take shape. We named the shape “Capton.©“
Captons, Guardians of the Brain
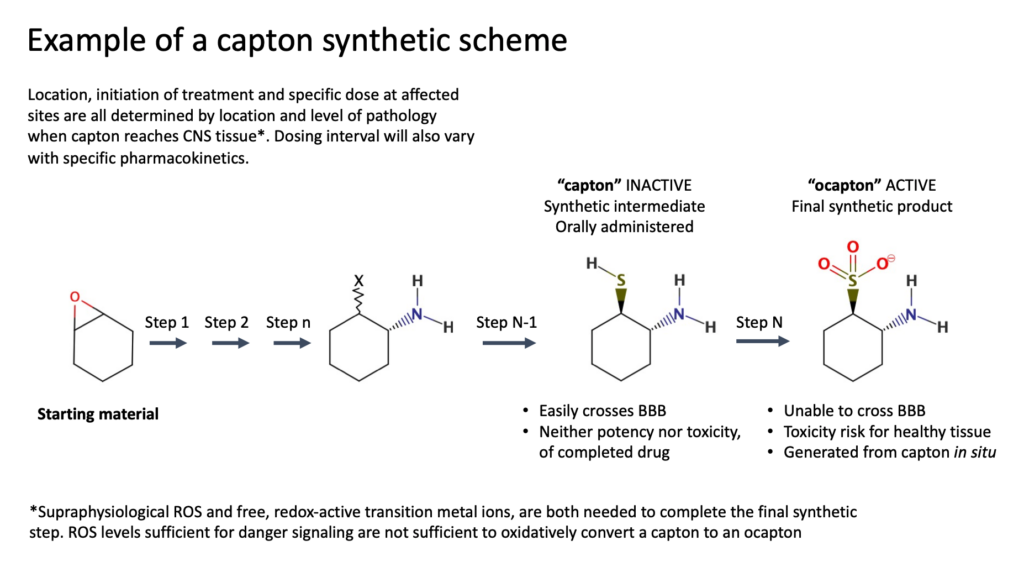
Captons are structurally modified small-molecule therapeutics that mitigate excitotoxicity, inflammation, and other mechanisms of CNS pathology. They are administered orally in an unfinished state, the penultimate intermediate of their synthetic route. Beyond providing the basis for structural pathosensitivity (oxidative capton activation), this approach improves drug transit across the blood-brain barrier by temporally reducing charge and polar surface area. When a capton encounters areas of damaged or dysfunctioning tissue in the brain, it reacts, formally completing drug synthesis while consuming three equivalents of ROS. More damage will bring more benefit. In contrast, silence will prevail in healthy subjects. Dependent on reactive metabolites generated by trauma, capton activation only occurs at locations where a therapeutic would bring benefit, and only in the CNS. Capton drugs are eliminated from the periphery without oxidative modification. This observation is consistent with well-established vascular physiology, where the composition of plasma in the periphery defines the composition of interstitial fluid. Strong redox buffering by human serum albumin (HSA) prevails in both compartments. HSA is undetectable in the brain interstitium. At steady-state equilibrium, about 80% of HSA bears a free thiol at cysteine-34 (Cys-34), equivalent to ~500 mM. ROS needed to convert capton to its active form is neutralized by HSA Cys-34. Consistent with this hypothesis, oxidized CPT-004 in plasma remains below the detection limit (~10 nM) during the 48-hour interval following intravenous administration. In the same subject, oxidized CPT-004 in distressed brain reaches levels over 5 μM.
Captons, Proof of Concept. Preclinical Pharmacokinetics and Pharmacodynamics
Enabled Therapeutics has completed studies on its lead capton (CPT-004). Reproducible data strongly support drug-like pharmacokinetics, widespread distribution throughout the brain at levels exceeding therapeutic, and irreversible activation under neuropathological conditions. In a seizure model, activation was determined to be nearly quantitative and strictly confined to distressed brain tissue. The performance of CPT-004 provides some justification for initiating a redefinition of the term “drug”, adding pathology-dependent dose-metering in situ to the list of design-based behaviors. New drugs following the capton example would act dynamically, generating active drug after administration and only when necessary.
Captons, Proof of Concept – Preclinical Efficacy
In 2017, Enabled Therapeutics was accepted into the National Institute of Neurological Disorders and Stroke (NIH-NINDS) Epilepsy Therapy Screening Program (ETSP). Among all anti-epileptic drugs currently sold, over half were selected in screens by the ETSP or its progenitor. CPT-004 was identified by the ETSP, based on reproducible evidence of seizure protection in the benchmark model. Ongoing collaborative efforts include characterization of CPT-004 in different models to assess durability of effect. Screening of other capton candidates also continues.
Captons, Treating Brain Trauma by Tackling Excitotoxicity and Inflammation
Enabled Therapeutics has directed its drug-development efforts toward epilepsy and traumatic brain injury. These conditions, as with almost all CNS ailments, are defined to a significant degree by excitotoxicity and inflammation. Excitotoxicity arises through the derangement of the brain’s fundamental process for neurotransmission, the communication of information between neurons. Compromise of cellular integrity releases glutamate, the primary excitatory neurotransmitter, into the synaptic and extra-synaptic extracellular spaces. While glial homeostatic mechanisms struggle to keep up, supraphysiological glutamate levels constitutively maintain AMPA and NMDA receptors in their active (“open”) form. Unable to reestablish ion gradients across the plasma membrane, neurons become flooded with calcium and die, releasing more glutamate. The pathology propagates outward. Excessive excitation places increasing demand on mitochondria, the main source of energy inside a cell and increase activation of NADPH oxidase, a plasma-membrane embedded complex that generates ROS (reactive oxygen species). Stressed mitochondria begin to falter in their execution of other tasks. The diminished ability to maintain calcium homeostasis is especially harmful. Mitochondria begin to fail, generating more ROS and triggering a variety of other pathogenic events, ultimately including the release of unchaperoned metal ions, including redox-active iron and copper. Captons bind metal ions and neutralize ROS during oxidative conversion to their bioactive form. Some captons specifically act to reduce excitation, countering neuropathological stress through multiple mechanisms. Inflammation arises through the dysfunctional behavior of non-neuronal cells, either from within the brain itself (glia) or imported from the periphery (T and B-lymphocytes). In healthy individuals, these cells ensure that neurons are healthy and functional. Neuronal damage releases a variety of toxic species along with glutamate. ROS and metal ions, the key components of capton activation, are especially well represented. The release of intracellular contents does not go unnoticed by the immune system. Some intracellular content is detected by microglia which react immediately to address apparent damage as well as signaling for additional help from throughout the brain and to key players from the periphery. The arrival of cells from outside the afflicted area, combined with swelling caused by loss of osmotic balance, results in inflammation and is accompanied by still more ROS. Enabled Therapeutics has selected exhaustive compound sets from public databases. Suitable compounds were bioactive and structurally configured for facile conversion to captons (“captonization”). Post-hoc analysis of the compounds indicated enrichment for anti-excitatory and anti-inflammatory potencies. This result informed Enabled Therapeutics’ initial choice of targets and their paired indications (see Pipeline). Both excitotoxicity and neuroinflammation share key features of pathophysiology relevant to COVID-19. By expanding our efforts in the lab, we would be able to resource programs to ease the suffering caused by the pandemic.
Captons, Competitive Advantages
• Blood-brain barrier transit: Captons cross the blood-brain barrier more easily than the therapeutic compounds from which they are derived.
• Injury-activated behavior: Captons generate neurotherapeutic drugs at the site and time of injury in amounts commensurate with the levels of pathology they encounter.
• Fewer side effects: Since captons selectively act in damaged areas of the brain, interference in healthy tissue function is obviated.
Captons, Partnering with the Pharma Industry
Captons are disruptive, a risk to the likelihood of acceptance by industry and, as a consequence, to the time it will take for the benefits of this potentially transformative technology to reach patients. We intend to partner with established pharma companies to ease the shock, securing uptake of our next-generation drug design by pushing most of the cost of technology into the post-commercialization era. Most current drugs on the market to treat TBI, CTE, seizures, and related conditions are handicapped by the indiscriminate flooding of the body with bioactivities needed to reach small areas of the brain. Captons release drug activity in appropriate amounts at discrete locations. In many cases, the enormous levels of a typical drug needed to achieve a small improvement in the brain come at the expense of high toxicity and intolerable side effects elsewhere.