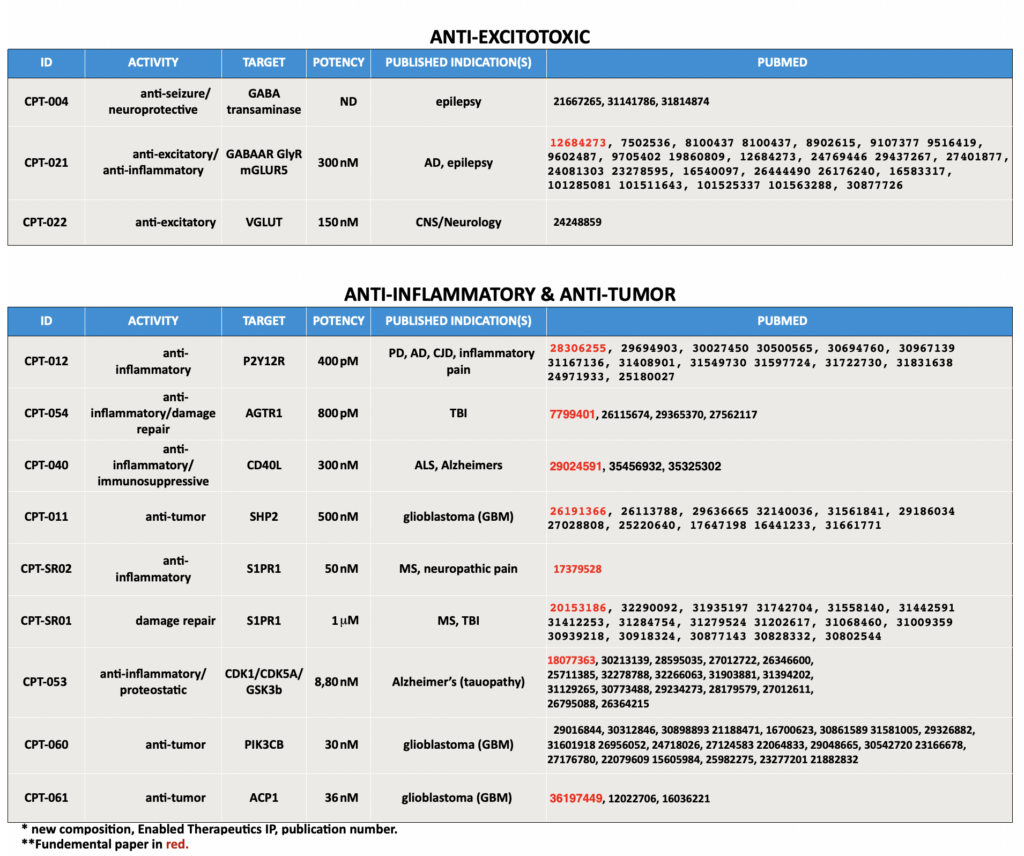
Enabled Therapeutics' Current Pipeline
Pre-Clinical Evidence in Animal Studies
Enabled Therapeutics has completed numerous efficacy studies demonstrating efficient capton distribution throughout the brain, conversion to a neuroprotectant upon excitotoxic insult, and protection from seizures.
Studies conducted by Charles River Laboratories
Data generated at Charles River Laboratories shows reproducible proof-of-concept (POC) for the generation of a neuroprotectant upon kainate-induced brain injury.
Good distribution of capton was detected inside the brain.
Transformation of the unfinished therapeutic to its active form was detected in injured tissue only, not in plasma or cerebrospinal fluid (CSF).
Studies conducted and funded by the National Institutes of Health
In 2017, Enabled Therapeutics was accepted to the National Institute of Neurological Disorders and Stroke (NIH-NINDS), Epilepsy Therapy
Screening Program (ETSP). The ETSP is a screening program for new anticonvulsive drugs.
ETSP showed reproducible POC demonstrating capton-dependent protection from seizure-induced by excitotoxic insult, identifying a lead capton from a series of candidates.
The NIH is testing the lead capton in additional models to assess the durability of effect.